 Megalomyrmex ants are chemical warriors, dispensing volatile venom alkaloids by
waving their stings
(i.e. gaster flagging)
as they enter another ant species' nest as parasites or
during competition (by predatory free-living species). Species of both lifestyles use gaster flagging as a
'warning shot', announcing their presence to hosts or competitors. If the host does not allow infiltration,
the invader will attack and kill. Infiltration can be accomplished as just described (i.e. chemical weaponry)
or through the alteration of cuticular hydrocarbons (i.e. surface chemistry) using chemical mimicry and/or
insignificance. Our work deciphers the chemical code of communication and manipulation used by parasites of
fungus-growing ants, linking behaviors observed to their chemical ecology by 1) testing three infiltration
strategy hypotheses with behavioral observations and chemical analysis, 2) identifying chemical compounds,
and 3) examining the results in an evolutionary context with phylogenetic hypothesis testing.
Megalomyrmex ants are chemical warriors, dispensing volatile venom alkaloids by
waving their stings
(i.e. gaster flagging)
as they enter another ant species' nest as parasites or
during competition (by predatory free-living species). Species of both lifestyles use gaster flagging as a
'warning shot', announcing their presence to hosts or competitors. If the host does not allow infiltration,
the invader will attack and kill. Infiltration can be accomplished as just described (i.e. chemical weaponry)
or through the alteration of cuticular hydrocarbons (i.e. surface chemistry) using chemical mimicry and/or
insignificance. Our work deciphers the chemical code of communication and manipulation used by parasites of
fungus-growing ants, linking behaviors observed to their chemical ecology by 1) testing three infiltration
strategy hypotheses with behavioral observations and chemical analysis, 2) identifying chemical compounds,
and 3) examining the results in an evolutionary context with phylogenetic hypothesis testing.
 Social organization of ant societies, from nestmate recognition to foraging,
is governed by complex chemical cues. This is especially true in the fungus-farming ants (Attini: Attina)
who must not only regulate behavior of other ants, but also fungal cultivars they have grown over millions
of years. Recent phylogenetic advances indicate several major evolutionary transitions in farming ant
societies, from colony-farms with less than 100 workers, to industrial-scale leaf-cutter ants whose
massive fungus gardens sustain millions of specialized workers. Fungal cultivars provide attines access
to a stable food source, but large standing resources (e.g., fungal crops and ant brood) also make the
ant farmers vulnerable to predators and thieves. We thus predicted that attine alarm systems have been
under intense selection. We identify attine alarm systems through observation and pheromone identification.
We observe large variation in behavior from aggressive resistance to passive tolerance. We are currently
interpreting our results in an evolutionary context to better understand how these traits evolve across
the fungus-growing ant tribe. Our results shed light on how attine ants cope with one of the central costs
of their farming lifestyle, and link chemistry, behavior and symbiotic coevolution.
Social organization of ant societies, from nestmate recognition to foraging,
is governed by complex chemical cues. This is especially true in the fungus-farming ants (Attini: Attina)
who must not only regulate behavior of other ants, but also fungal cultivars they have grown over millions
of years. Recent phylogenetic advances indicate several major evolutionary transitions in farming ant
societies, from colony-farms with less than 100 workers, to industrial-scale leaf-cutter ants whose
massive fungus gardens sustain millions of specialized workers. Fungal cultivars provide attines access
to a stable food source, but large standing resources (e.g., fungal crops and ant brood) also make the
ant farmers vulnerable to predators and thieves. We thus predicted that attine alarm systems have been
under intense selection. We identify attine alarm systems through observation and pheromone identification.
We observe large variation in behavior from aggressive resistance to passive tolerance. We are currently
interpreting our results in an evolutionary context to better understand how these traits evolve across
the fungus-growing ant tribe. Our results shed light on how attine ants cope with one of the central costs
of their farming lifestyle, and link chemistry, behavior and symbiotic coevolution.
 Chemical communication is fundamental and highly complex in social insect societies. Ants
in particular employ a remarkable diversity of compounds (i.e., semiochemicals) to maintain social cohesion
among nestmates and gain essential resources through coordinated foraging. Although the compounds used can be
functionally specific, they are vulnerable to exploitation by eavesdropping natural enemies (e.g., parasitoids,
aphids, butterflies, ants, beetles, frogs). Ant societies consist of a collection of resources that warrant
avid defense systems and yet even the largest hideouts (e.g., underground nests of Atta leaf-cutting species)
are invaded. A great deal is known about numerous ant colony invaders but how these organisms locate host
colonies—including what chemical signals are utilized—and the costs of exploitation, are still poorly understood.
Our objective is to understand how ant communication systems get “hacked” and determine ways these systems
can be investigated. We explore (1) traits that make species vulnerable to exploitation, (2) counter defenses
against predators and parasites, and (3) fundamental gaps in our understanding of chemical eavesdropping by ant
enemies, and strategies to advance this area of research. These insights will shed light on the modulation of
signals as products of antagonistic selection between exploiters and their victims.
Chemical communication is fundamental and highly complex in social insect societies. Ants
in particular employ a remarkable diversity of compounds (i.e., semiochemicals) to maintain social cohesion
among nestmates and gain essential resources through coordinated foraging. Although the compounds used can be
functionally specific, they are vulnerable to exploitation by eavesdropping natural enemies (e.g., parasitoids,
aphids, butterflies, ants, beetles, frogs). Ant societies consist of a collection of resources that warrant
avid defense systems and yet even the largest hideouts (e.g., underground nests of Atta leaf-cutting species)
are invaded. A great deal is known about numerous ant colony invaders but how these organisms locate host
colonies—including what chemical signals are utilized—and the costs of exploitation, are still poorly understood.
Our objective is to understand how ant communication systems get “hacked” and determine ways these systems
can be investigated. We explore (1) traits that make species vulnerable to exploitation, (2) counter defenses
against predators and parasites, and (3) fundamental gaps in our understanding of chemical eavesdropping by ant
enemies, and strategies to advance this area of research. These insights will shed light on the modulation of
signals as products of antagonistic selection between exploiters and their victims.
 Ants possess many exocrine glands that produce a variety of compounds important
for chemical communication. These complex chemosensory systems often involve a mixture of small volatile
molecules for within- and between-species communication (i.e. semiochemicals). We focus on determining the identity and
function of semiochemicals found in species of two Myrmicinae ant tribes, Solenopsidini and Attini.
These tribes include Megalomyrmex and fungus-growing ants, respectively. The overall objective
is to understand the diversity of compounds used in ant communication from an evolutionary perspective and
relate them to symbiotic interactions while determining there adaptive significance.
Ants possess many exocrine glands that produce a variety of compounds important
for chemical communication. These complex chemosensory systems often involve a mixture of small volatile
molecules for within- and between-species communication (i.e. semiochemicals). We focus on determining the identity and
function of semiochemicals found in species of two Myrmicinae ant tribes, Solenopsidini and Attini.
These tribes include Megalomyrmex and fungus-growing ants, respectively. The overall objective
is to understand the diversity of compounds used in ant communication from an evolutionary perspective and
relate them to symbiotic interactions while determining there adaptive significance.
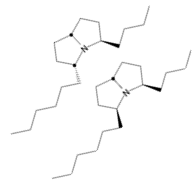
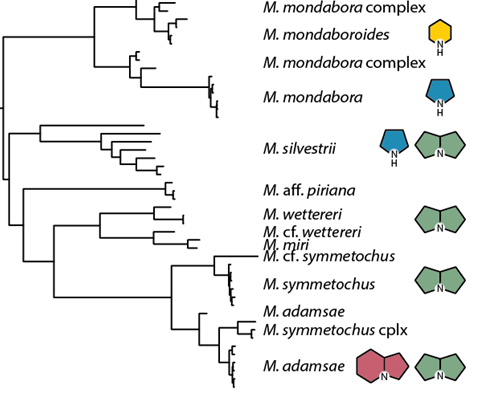 Social parasites exploit other societies by invading and stealing resources. Megalomyrmex
ants enter protected fungus-growing ant nests using chemical weaponry made from alkaloid-based venom. Just as in
closely related Solenopsis and Monomorium genera, Megalomyrmex ants have pyrrolidines, pyrrolizidines,
piperidines, and pyrrolines in their venom. By characterizing the venom alkaloids of many species with different
lifestyles (e.g., parasitic, predatory, etc.) and describing their function, we will elucidate their origin and
diversification across ants. We are currently (1) gathering samples from Central and South America, (2)
conducting behavioral assays to determine communication function, (3) using microbial bioassays to evaluate
antimicrobial properties, and (4) constructing an ultraconserved element phylogeny of the Megalomyrmex genus.
Social parasites exploit other societies by invading and stealing resources. Megalomyrmex
ants enter protected fungus-growing ant nests using chemical weaponry made from alkaloid-based venom. Just as in
closely related Solenopsis and Monomorium genera, Megalomyrmex ants have pyrrolidines, pyrrolizidines,
piperidines, and pyrrolines in their venom. By characterizing the venom alkaloids of many species with different
lifestyles (e.g., parasitic, predatory, etc.) and describing their function, we will elucidate their origin and
diversification across ants. We are currently (1) gathering samples from Central and South America, (2)
conducting behavioral assays to determine communication function, (3) using microbial bioassays to evaluate
antimicrobial properties, and (4) constructing an ultraconserved element phylogeny of the Megalomyrmex genus.
 Megalomyrmex Forel (Myrmicinae: Solenopsidini) consists of 44 species with diverse life
history strategies. Most species are predatory and may also tend honeydew-producing insects. A morphologically
derived group of species are social parasites that consume the brood and fungus garden within fungus-growing ant
nests. The reproductive strategies of Megalomyrmex queens are somewhat aligned with these life-style patterns.
Predatory species in the leoninus species group are large and have ergatoid (i.e. permanently wingless) queens,
whereas the social parasitic species are smaller and typically have winged queens. We examine the evolution of
queen reproductive strategies in Megalomyrmex and other Solenopsidini ant species using phylogenetic and
morphometric methods.
Megalomyrmex Forel (Myrmicinae: Solenopsidini) consists of 44 species with diverse life
history strategies. Most species are predatory and may also tend honeydew-producing insects. A morphologically
derived group of species are social parasites that consume the brood and fungus garden within fungus-growing ant
nests. The reproductive strategies of Megalomyrmex queens are somewhat aligned with these life-style patterns.
Predatory species in the leoninus species group are large and have ergatoid (i.e. permanently wingless) queens,
whereas the social parasitic species are smaller and typically have winged queens. We examine the evolution of
queen reproductive strategies in Megalomyrmex and other Solenopsidini ant species using phylogenetic and
morphometric methods.
 Symbioses are inherently complex, involving multiple selective pressures from various organisms
that influence the evolution of semiochemicals in social insects. This complexity can create methodological
challenges that impede progress in understanding function and the adaptive significance of ant-derived chemicals.
We have discovered that Megalomyrmex social parasites and their fungus-growing ant hosts share a subset of bacterial
symbionts, primarily consisting of Entomoplasmatales, Bartonellaceae, Acinetobacter, Wolbachia and Pseudonocardia
and that Entomoplasmatales and Bartonellaceae can co-infect specifically associated combinations of hosts and
social parasites. We are currently interested in the bacteria found in the fungus-garden and how the parasite
venom influences the prevalence of helpful microbes in the fungus-growing ant symbiotic network.
Symbioses are inherently complex, involving multiple selective pressures from various organisms
that influence the evolution of semiochemicals in social insects. This complexity can create methodological
challenges that impede progress in understanding function and the adaptive significance of ant-derived chemicals.
We have discovered that Megalomyrmex social parasites and their fungus-growing ant hosts share a subset of bacterial
symbionts, primarily consisting of Entomoplasmatales, Bartonellaceae, Acinetobacter, Wolbachia and Pseudonocardia
and that Entomoplasmatales and Bartonellaceae can co-infect specifically associated combinations of hosts and
social parasites. We are currently interested in the bacteria found in the fungus-garden and how the parasite
venom influences the prevalence of helpful microbes in the fungus-growing ant symbiotic network.
 Species identification is an essential component of any scientific endeavor.
In our work we are constantly faced with the question: Is this species what I think it is? We are committed
to advancing our understanding of ant evolution by contributing to species descriptions and supporting
collaborations with skilled taxonomists. Through collaborations with South American scientists, we are
working on revising the Megalomyrmex genus, focusing on Central America,
Columbia, and Brazil.
Species identification is an essential component of any scientific endeavor.
In our work we are constantly faced with the question: Is this species what I think it is? We are committed
to advancing our understanding of ant evolution by contributing to species descriptions and supporting
collaborations with skilled taxonomists. Through collaborations with South American scientists, we are
working on revising the Megalomyrmex genus, focusing on Central America,
Columbia, and Brazil.
 Megalomyrmex ants are chemical warriors, dispensing volatile venom alkaloids by
waving their stings
(i.e. gaster flagging)
as they enter another ant species' nest as parasites or
during competition (by predatory free-living species). Species of both lifestyles use gaster flagging as a
'warning shot', announcing their presence to hosts or competitors. If the host does not allow infiltration,
the invader will attack and kill. Infiltration can be accomplished as just described (i.e. chemical weaponry)
or through the alteration of cuticular hydrocarbons (i.e. surface chemistry) using chemical mimicry and/or
insignificance. Our work deciphers the chemical code of communication and manipulation used by parasites of
fungus-growing ants, linking behaviors observed to their chemical ecology by 1) testing three infiltration
strategy hypotheses with behavioral observations and chemical analysis, 2) identifying chemical compounds,
and 3) examining the results in an evolutionary context with phylogenetic hypothesis testing.
Megalomyrmex ants are chemical warriors, dispensing volatile venom alkaloids by
waving their stings
(i.e. gaster flagging)
as they enter another ant species' nest as parasites or
during competition (by predatory free-living species). Species of both lifestyles use gaster flagging as a
'warning shot', announcing their presence to hosts or competitors. If the host does not allow infiltration,
the invader will attack and kill. Infiltration can be accomplished as just described (i.e. chemical weaponry)
or through the alteration of cuticular hydrocarbons (i.e. surface chemistry) using chemical mimicry and/or
insignificance. Our work deciphers the chemical code of communication and manipulation used by parasites of
fungus-growing ants, linking behaviors observed to their chemical ecology by 1) testing three infiltration
strategy hypotheses with behavioral observations and chemical analysis, 2) identifying chemical compounds,
and 3) examining the results in an evolutionary context with phylogenetic hypothesis testing.
 Social organization of ant societies, from nestmate recognition to foraging,
is governed by complex chemical cues. This is especially true in the fungus-farming ants (Attini: Attina)
who must not only regulate behavior of other ants, but also fungal cultivars they have grown over millions
of years. Recent phylogenetic advances indicate several major evolutionary transitions in farming ant
societies, from colony-farms with less than 100 workers, to industrial-scale leaf-cutter ants whose
massive fungus gardens sustain millions of specialized workers. Fungal cultivars provide attines access
to a stable food source, but large standing resources (e.g., fungal crops and ant brood) also make the
ant farmers vulnerable to predators and thieves. We thus predicted that attine alarm systems have been
under intense selection. We identify attine alarm systems through observation and pheromone identification.
We observe large variation in behavior from aggressive resistance to passive tolerance. We are currently
interpreting our results in an evolutionary context to better understand how these traits evolve across
the fungus-growing ant tribe. Our results shed light on how attine ants cope with one of the central costs
of their farming lifestyle, and link chemistry, behavior and symbiotic coevolution.
Social organization of ant societies, from nestmate recognition to foraging,
is governed by complex chemical cues. This is especially true in the fungus-farming ants (Attini: Attina)
who must not only regulate behavior of other ants, but also fungal cultivars they have grown over millions
of years. Recent phylogenetic advances indicate several major evolutionary transitions in farming ant
societies, from colony-farms with less than 100 workers, to industrial-scale leaf-cutter ants whose
massive fungus gardens sustain millions of specialized workers. Fungal cultivars provide attines access
to a stable food source, but large standing resources (e.g., fungal crops and ant brood) also make the
ant farmers vulnerable to predators and thieves. We thus predicted that attine alarm systems have been
under intense selection. We identify attine alarm systems through observation and pheromone identification.
We observe large variation in behavior from aggressive resistance to passive tolerance. We are currently
interpreting our results in an evolutionary context to better understand how these traits evolve across
the fungus-growing ant tribe. Our results shed light on how attine ants cope with one of the central costs
of their farming lifestyle, and link chemistry, behavior and symbiotic coevolution.
 Chemical communication is fundamental and highly complex in social insect societies. Ants
in particular employ a remarkable diversity of compounds (i.e., semiochemicals) to maintain social cohesion
among nestmates and gain essential resources through coordinated foraging. Although the compounds used can be
functionally specific, they are vulnerable to exploitation by eavesdropping natural enemies (e.g., parasitoids,
aphids, butterflies, ants, beetles, frogs). Ant societies consist of a collection of resources that warrant
avid defense systems and yet even the largest hideouts (e.g., underground nests of Atta leaf-cutting species)
are invaded. A great deal is known about numerous ant colony invaders but how these organisms locate host
colonies—including what chemical signals are utilized—and the costs of exploitation, are still poorly understood.
Our objective is to understand how ant communication systems get “hacked” and determine ways these systems
can be investigated. We explore (1) traits that make species vulnerable to exploitation, (2) counter defenses
against predators and parasites, and (3) fundamental gaps in our understanding of chemical eavesdropping by ant
enemies, and strategies to advance this area of research. These insights will shed light on the modulation of
signals as products of antagonistic selection between exploiters and their victims.
Chemical communication is fundamental and highly complex in social insect societies. Ants
in particular employ a remarkable diversity of compounds (i.e., semiochemicals) to maintain social cohesion
among nestmates and gain essential resources through coordinated foraging. Although the compounds used can be
functionally specific, they are vulnerable to exploitation by eavesdropping natural enemies (e.g., parasitoids,
aphids, butterflies, ants, beetles, frogs). Ant societies consist of a collection of resources that warrant
avid defense systems and yet even the largest hideouts (e.g., underground nests of Atta leaf-cutting species)
are invaded. A great deal is known about numerous ant colony invaders but how these organisms locate host
colonies—including what chemical signals are utilized—and the costs of exploitation, are still poorly understood.
Our objective is to understand how ant communication systems get “hacked” and determine ways these systems
can be investigated. We explore (1) traits that make species vulnerable to exploitation, (2) counter defenses
against predators and parasites, and (3) fundamental gaps in our understanding of chemical eavesdropping by ant
enemies, and strategies to advance this area of research. These insights will shed light on the modulation of
signals as products of antagonistic selection between exploiters and their victims.
 Ants possess many exocrine glands that produce a variety of compounds important
for chemical communication. These complex chemosensory systems often involve a mixture of small volatile
molecules for within- and between-species communication (i.e. semiochemicals). We focus on determining the identity and
function of semiochemicals found in species of two Myrmicinae ant tribes, Solenopsidini and Attini.
These tribes include Megalomyrmex and fungus-growing ants, respectively. The overall objective
is to understand the diversity of compounds used in ant communication from an evolutionary perspective and
relate them to symbiotic interactions while determining there adaptive significance.
Ants possess many exocrine glands that produce a variety of compounds important
for chemical communication. These complex chemosensory systems often involve a mixture of small volatile
molecules for within- and between-species communication (i.e. semiochemicals). We focus on determining the identity and
function of semiochemicals found in species of two Myrmicinae ant tribes, Solenopsidini and Attini.
These tribes include Megalomyrmex and fungus-growing ants, respectively. The overall objective
is to understand the diversity of compounds used in ant communication from an evolutionary perspective and
relate them to symbiotic interactions while determining there adaptive significance.
 Social parasites exploit other societies by invading and stealing resources. Megalomyrmex
ants enter protected fungus-growing ant nests using chemical weaponry made from alkaloid-based venom. Just as in
closely related Solenopsis and Monomorium genera, Megalomyrmex ants have pyrrolidines, pyrrolizidines,
piperidines, and pyrrolines in their venom. By characterizing the venom alkaloids of many species with different
lifestyles (e.g., parasitic, predatory, etc.) and describing their function, we will elucidate their origin and
diversification across ants. We are currently (1) gathering samples from Central and South America, (2)
conducting behavioral assays to determine communication function, (3) using microbial bioassays to evaluate
antimicrobial properties, and (4) constructing an ultraconserved element phylogeny of the Megalomyrmex genus.
Social parasites exploit other societies by invading and stealing resources. Megalomyrmex
ants enter protected fungus-growing ant nests using chemical weaponry made from alkaloid-based venom. Just as in
closely related Solenopsis and Monomorium genera, Megalomyrmex ants have pyrrolidines, pyrrolizidines,
piperidines, and pyrrolines in their venom. By characterizing the venom alkaloids of many species with different
lifestyles (e.g., parasitic, predatory, etc.) and describing their function, we will elucidate their origin and
diversification across ants. We are currently (1) gathering samples from Central and South America, (2)
conducting behavioral assays to determine communication function, (3) using microbial bioassays to evaluate
antimicrobial properties, and (4) constructing an ultraconserved element phylogeny of the Megalomyrmex genus.
 Megalomyrmex Forel (Myrmicinae: Solenopsidini) consists of 44 species with diverse life
history strategies. Most species are predatory and may also tend honeydew-producing insects. A morphologically
derived group of species are social parasites that consume the brood and fungus garden within fungus-growing ant
nests. The reproductive strategies of Megalomyrmex queens are somewhat aligned with these life-style patterns.
Predatory species in the leoninus species group are large and have ergatoid (i.e. permanently wingless) queens,
whereas the social parasitic species are smaller and typically have winged queens. We examine the evolution of
queen reproductive strategies in Megalomyrmex and other Solenopsidini ant species using phylogenetic and
morphometric methods.
Megalomyrmex Forel (Myrmicinae: Solenopsidini) consists of 44 species with diverse life
history strategies. Most species are predatory and may also tend honeydew-producing insects. A morphologically
derived group of species are social parasites that consume the brood and fungus garden within fungus-growing ant
nests. The reproductive strategies of Megalomyrmex queens are somewhat aligned with these life-style patterns.
Predatory species in the leoninus species group are large and have ergatoid (i.e. permanently wingless) queens,
whereas the social parasitic species are smaller and typically have winged queens. We examine the evolution of
queen reproductive strategies in Megalomyrmex and other Solenopsidini ant species using phylogenetic and
morphometric methods.
 Symbioses are inherently complex, involving multiple selective pressures from various organisms
that influence the evolution of semiochemicals in social insects. This complexity can create methodological
challenges that impede progress in understanding function and the adaptive significance of ant-derived chemicals.
We have discovered that Megalomyrmex social parasites and their fungus-growing ant hosts share a subset of bacterial
symbionts, primarily consisting of Entomoplasmatales, Bartonellaceae, Acinetobacter, Wolbachia and Pseudonocardia
and that Entomoplasmatales and Bartonellaceae can co-infect specifically associated combinations of hosts and
social parasites. We are currently interested in the bacteria found in the fungus-garden and how the parasite
venom influences the prevalence of helpful microbes in the fungus-growing ant symbiotic network.
Symbioses are inherently complex, involving multiple selective pressures from various organisms
that influence the evolution of semiochemicals in social insects. This complexity can create methodological
challenges that impede progress in understanding function and the adaptive significance of ant-derived chemicals.
We have discovered that Megalomyrmex social parasites and their fungus-growing ant hosts share a subset of bacterial
symbionts, primarily consisting of Entomoplasmatales, Bartonellaceae, Acinetobacter, Wolbachia and Pseudonocardia
and that Entomoplasmatales and Bartonellaceae can co-infect specifically associated combinations of hosts and
social parasites. We are currently interested in the bacteria found in the fungus-garden and how the parasite
venom influences the prevalence of helpful microbes in the fungus-growing ant symbiotic network.
 Species identification is an essential component of any scientific endeavor.
In our work we are constantly faced with the question: Is this species what I think it is? We are committed
to advancing our understanding of ant evolution by contributing to species descriptions and supporting
collaborations with skilled taxonomists. Through collaborations with South American scientists, we are
working on revising the Megalomyrmex genus, focusing on Central America,
Columbia, and Brazil.
Species identification is an essential component of any scientific endeavor.
In our work we are constantly faced with the question: Is this species what I think it is? We are committed
to advancing our understanding of ant evolution by contributing to species descriptions and supporting
collaborations with skilled taxonomists. Through collaborations with South American scientists, we are
working on revising the Megalomyrmex genus, focusing on Central America,
Columbia, and Brazil.